Module 2: Therapeutics Clinical Implementation Guide Products for Treatment of Mild-to-Moderate COVID-19
On this page:
- Preferred Therapies per NIH Guidelines
- Alternative Therapies
Paxlovid (nirmatrelvir co-packaged with ritonavir)
Pfizer Oral Antiviral (Preferred)
Emergency Use Authorization (EUA) (while supply remains) and NDA labeled Paxlovid are available.
Paxlovid Product Information
FDA Fact Sheets and Prescribing Information for Paxlovid
Paxlovid Manufacturer's Resources
Paxlovid Prescribing Resources
Safety Reporting
Other Resources
Paxlovid Emergency Use Authorization (EUA)
Paxlovid EUA is authorized for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg), who are at high risk for progression to severe COVID-19, including hospitalization or death.
Initiate as soon as possible after diagnosis of COVID-19 and within 5 days of symptom onset.
Paxlovid includes: nirmatrelvir (a SARS-CoV-2 main proteases inhibitor) co-packaged with ritonavir (a CYP34A inhibitor).
Paxlovid is not authorized for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19.
Paxlovid (nirmatrelvir co-packaged with ritonavir) may be prescribed for an individual patient by physicians, advanced practice registered nurses, and physician assistants that are licensed or authorized under state law to prescribe drugs in the therapeutic class to which Paxlovid (nirmatrelvir co-packaged ritonavir) belongs (i.e., anti-infectives). Pharmacists are also authorized under the EUA to prescribe Paxlovid under certain conditions.
Paxlovid FDA Approval
On May 25, 2023, FDA approved a New Drug Application (NDA) for Paxlovid for the treatment of mild to moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death.
Paxlovid also remains authorized under EUA to ensure continued access for all eligible patients to the current supply of Paxlovid, including adult patients.
The EUA continues to authorize Paxlovid to treat certain eligible pediatric patients, a patient population that is not covered under the approved NDA for Paxlovid at this time.
NDA-labeled Paxlovid (nirmatrelvir tablets and ritonavir tablets) continues to be available from the federal government at no cost for federal entities.
Paxlovid Pharmacist Prescribing Authorization under EUA
As outlined in the EUA, Paxlovid may also be prescribed for an individual patient by a state-licensed pharmacist under the following conditions:
- Sufficient information is available, such as through access to health records less than 12 months old or consultation with a health care provider in an established provider-patient relationship with the individual patient, to assess renal and hepatic function; and
- Sufficient information is available, such as through access to health records, patient reporting of medical history, or consultation with a health care provider in an established provider-patient relationship with the individual patient, to obtain a comprehensive list of medications (prescribed and non-prescribed) that the patient is taking to assess for potential drug interaction.
The state-licensed pharmacist should refer an individual patient for clinical evaluation (e.g., telehealth, in-person visit) with a physician, advanced practice registered nurse, or physician assistant licensed or authorized under state law to prescribe drugs, if any of the following apply:
- Sufficient information is not available to assess renal and hepatic function.
- Sufficient information is not available to assess for a potential drug interaction.
- Modification of other medications is recommended due to a potential drug interaction.
- Paxlovid is not an appropriate therapeutic option based on the authorized Fact Sheet for Healthcare Providers or due to potential drug interactions for which recommended monitoring would not be feasible.
Paxlovid Dosage and Administration
- eGFR > 60 mL/min: 300 mg nirmatrelvir (two 150 mg tablets) co-packaged with 100 mg ritonavir (one 100 mg tablet) taken together twice daily for 5 days.
- Dose reduction for moderate renal impairment eGFR > 30 mL/min to < 60 mL/min: 150 mg nirmatrelvir (one 150 mg tablet) co-packaged with 100 mg ritonavir (one 100 mg tablet) taken together twice daily for 5 days.
- eGFR <30 mL/min: currently not recommended.
- Severe hepatic impairment (Child-Pugh Class C): currently not recommended.
Paxlovid Contraindications and Precautions
- History of clinically significant hypersensitivity reactions to the active ingredients or any other components.
- Co-administration with drugs highly dependent on CYP3A for clearance may result in life-threatening reactions (see the Liverpool Covid-19 interaction checker).
- Co-administration with potent CYP3A inducers may result in reduced nirmatrelvir plasma concentrations and potential loss of virologic response.
- The concomitant use of Paxlovid (nirmatrelvir co-packaged with ritonavir) and certain other drugs may result in potentially significant drug interactions.
- Hepatic transaminase elevations, hypersensitivity reactions, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir.
- Paxlovid (nirmatrelvir co-packaged with ritonavir) use may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection.
Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers
Medical History
- Mild to moderate COVID-19 with symptom onset within 5 days
- Age ≥ 12 years of age and weighing at least 40 kg (if utilizing product under EUA)
- Adult (if utilizing approved product)
- Has one or more risk factors for progression to severe COVID-19
- Not requiring hospitalization due to severe or critical COVID-19 at treatment initiation
- No known or suspected severe renal impairment (eGFR < 30 mL/min)
- No known or suspected severe hepatic impairment
- No history of clinically significant hypersensitivity reactions to the active ingredients (nirmatrelvir or ritonavir) or other components of the product
Concomitant Medications
- Assess patient's home medication list for drug-drug interactions
- See the table in Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers
- HMG-CoA reductase inhibitors (statins)
- Patient is taking lovastatin or simvastatin, which are contraindicated with Paxlovid coadministration: the statin can be held 12 hours prior to the first dose of Paxlovid treatment, held during the 5 days of treatment, and restarted 5 days after completing Paxlovid.
- Patient is taking atorvastatin or rosuvastatin: temporary discontinuation of atorvastatin and rosuvastatin during treatment with Paxlovid should be considered depending on statin dose. Atorvastatin and rosuvastatin do not need to be held prior to or after completing Paxlovid.
- Hormonal contraceptives containing ethinyl estradiol:
- Patient is taking a hormonal contraceptive containing ethinyl estradiol: the need for an additional non-hormonal method of contraception during the 5 days of Paxlovid treatment and until one menstrual cycle after stopping Paxlovid should be recommended.
- Medications for HIV-1 Treatment:
- Patient is taking medications for the treatment of HIV-1 infection: With the exception of maraviroc, HIV antiretroviral medications can be co-administered with Paxlovid without dose adjustment, but arranging follow-up by the HIV care provider to monitor for side effects is recommended.
- Other Drugs with Established and Other Potentially Significant Drug Interactions with Paxlovid
Prescriber is encouraged to include a note to the pharmacist in the prescription stating:
Please fill prescription by __________[insert date]_________. This prescription fill by date is within 5 days from symptom onset and complies with the patient eligibility criteria under the EUA.
Paxlovid Patient Eligibility Screening Checklist Tools for Prescribers
Other Drugs with Established and Other Potentially Significant Drug Interactions with PAXLOVID (listed alphabetically by generic name).
Interaction Codes:

Coadministration of this drug with PAXLOVID is CONTRAINDICATED. For further information, refer to the Fact Sheet for Healthcare Providers and the Individual Prescribing for the drug.

Coadministration of this drug with PAXLOVID should be avoided and/or holding of the drug, dose adjustment of this drug, or special monitoring is necessary. Consultation with the prescriber of the potentially interacting drug is recommended. For further information, refer to the Health Care Provider Fact Sheet and the individual Prescribing Information for the drug.
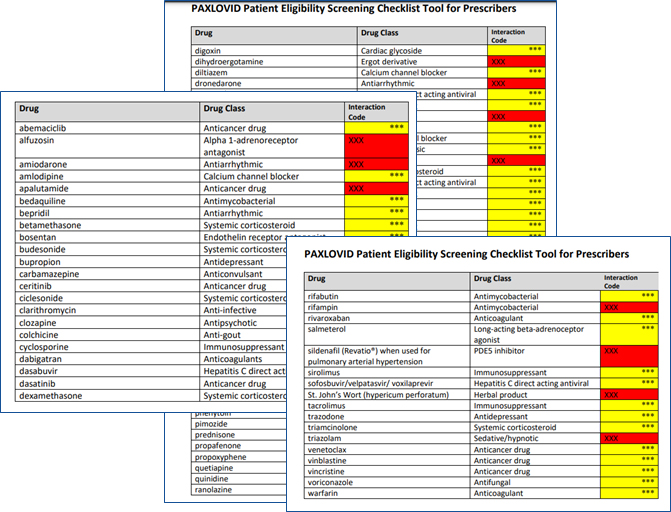
To learn more, see table in Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers
Paxlovid Formulation and Packaging
Paxlovid EUA Product

Standard Dose
300 mg nirmatrelvir; 100 mg ritonavir: Each carton contains 30 tablets divided in 5 daily dose blister cards. Each blister card contains 4 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each). Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
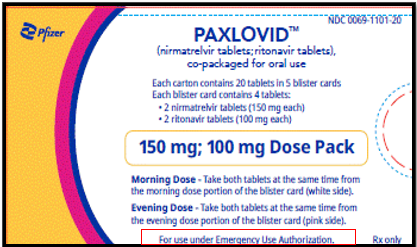
Reduced Dose (Renal)
150 mg nirmatrelvir; 100 mg ritonavir: Each carton contains 20 tablets divided in 5 daily dose blister cards. Each blister card contains 2 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each). Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
Note: EUA-labeled Paxlovid will no longer be authorized for emergency use after March 8, 2024.
Commercial NDA-labeled

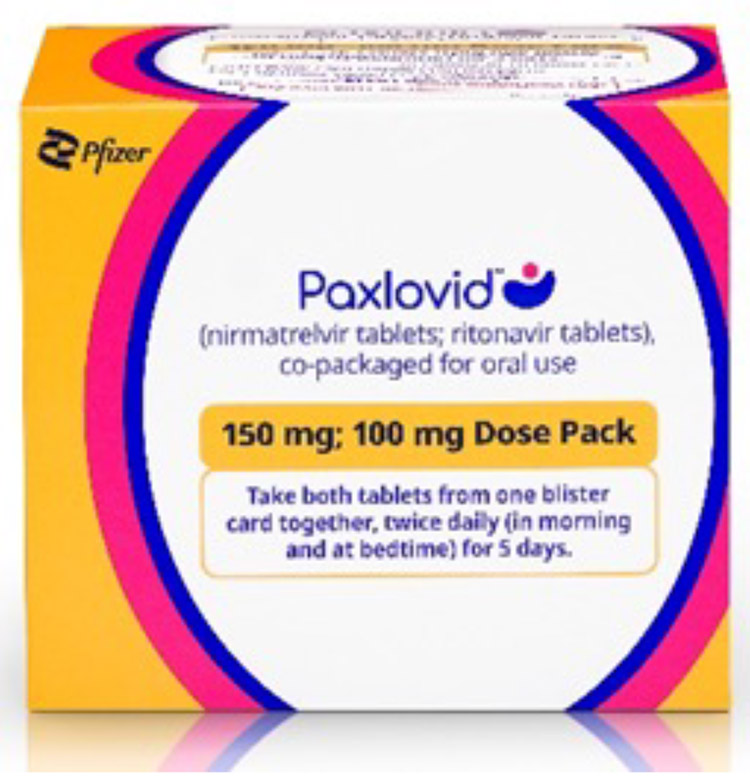
Standard Dose
300 mg nirmatrelvir; 100 mg ritonavir: Each carton contains 30 tablets divided in 5 daily dose blister cards. Each blister card contains 4 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each). Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
Reduced Dose (Renal)
150 mg nirmatrelvir; 100 mg ritonavir: Each carton contains 20 tablets divided in 5 daily dose blister cards. Each blister card contains 2 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each). Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
Viral RNA Rebound
Post-treatment increases in SARS-CoV-2 RNA levels (i.e., viral RNA rebound) in nasopharyngeal samples were observed on Day 10 and/or Day 14 in a subset of Paxlovid and placebo recipients, irrespective of COVID-19 symptoms. The frequency of detection of post-treatment viral RNA rebound varied according to analysis parameters but was generally similar among Paxlovid and placebo recipients, regardless of the rebound definition used. A similar or smaller percentage of placebo recipients compared to Paxlovid recipients had nasopharyngeal viral RNA results < LLOQ (lower limit of quantitation) at all study timepoints in both the treatment and post-treatment periods.
Post-treatment viral RNA rebound was not associated with the primary clinical outcome of COVID-19-related hospitalization or death from any cause through Day 28 following the single 5-day course of Paxlovid treatment. Post-treatment viral RNA rebound also was not associated with drug resistance as measured by Mpro sequencing. The clinical relevance or exact incidence of post-treatment increases in viral RNA following Paxlovid or placebo treatment is unknown at this time.
Viral RNA rebound* is not specific to Paxlovid or Lagevrio and has been seen in placebo recipients.
* Not exclusive to Paxlovid
For more information see the Microbiology (12.4) section of the Fact Sheet for Healthcare Providers: EUA for Paxlovid
Paxlovid Product Information & Ordering Logistics
EUA Status: EUA for pediatric patients (12 years of age and older weighing at least 40 kg) is active: NDA-labeled Paxlovid will be used—Active
Procurement: USG purchased and commercially available.
Eligibility information for product labeled under EUA:
Indications and usage for product labeled under FDA approval
To order Paxlovid: Process for Ordering COVID-19 Therapeutics | HHS/ASPR
Where to find Paxlovid Treatments Locator (hhs.gov)
Veklury (remdesivir)
Gilead Antiviral for IV Infusion (Preferred)
Veklury Product Information
Vekury Prescribing Information & FDA Fact Sheets
Veklury Manufacturer's Resources
Veklury Safety Reporting
Veklury Outpatient Use
FDA approved Veklury (remdesivir) for certain non-hospitalized adults and pediatric patients for treatment of mild-to-moderate COVID-19, including:
- Adults and pediatric patients 28 days of age and older and weighing at least 3 kg, AND
- Who are not hospitalized and have mild-to-moderate COVID-19 and are at high risk for progression to severe COVID-19, including hospitalization or death
The treatment course of Veklury (remdesivir) should be initiated as soon as possible after diagnosis of symptomatic COVID-19 has been made and within 7 days of symptom onset. The recommended total duration of treatment for non-hospitalized patients is 3 days.
No dosage adjustment of Veklury is recommended in patients with any degree of renal impairment, including those on dialysis for adult and pediatric patients.
- Veklury may be administered without regard to the timing of dialysis in all patients.
No dosage adjustment of Veklury is recommended for patients with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, or C). The label still recommends:
- Initial hepatic laboratory testing in all patients, before starting Veklury and during treatment as clinically appropriate
- Discontinuation be considered if alanine transaminase (ALT) levels increase to 10 times the upper limit of normal or if ALT elevation is accompanied by signs or symptoms of liver inflammation.
Veklury Dosage and Administration
Dosage:
- For adults and pediatric patients who weight > 40kg: 200 mg on Day 1, followed by once-daily maintenance doses of 100 mg from Day 2 administered only via intravenous infusion over 30 to 120 minutes.
- For pediatric patients 28 days of age and older and weighing 3 kg to less than 40 kg: 5 mg/kg on Day 1 followed by 5 mg/kg once daily from Day 2.
Dosage Forms:
- For injection: 100 mg of remdesivir as a lyophilized powder, in a single-dose vial
- Injection: 100 mg/20 mL (5 mg/mL) remdesivir, in a single-dose vial
Veklury Contraindications and Precautions
Contraindications
- History of clinically significant hypersensitivity reactions to Veklury or any components of the product
- Hypersensitivity including infusion-related and anaphylactic reactions
Pre-administration testing
- Perform hepatic laboratory testing in all patients before starting Veklury and during treatment as clinically appropriate
- Assess prothrombin time before starting Veklury and monitor as clinically appropriate
Increased risk of transaminase elevations with administration
Risk of reduced antiviral activity when co-administered with chloroquine phosphate or hydroxychloroquine sulfate
Veklury Pediatric Information
Veklury Pediatric Eligibility Criteria
- Pediatric patients 28 Days of Age and Older and weighing 3 kg to less than 40 kg*
- Mild-to-moderate COVID-19 and at high risk for progression to severe disease
- Initiate within 7 days of symptom onset
* Pediatric patients less than 12 years of age but weighing 40kg or greater receive Veklury at the adult dose
Veklury Pediatric Dosing and Administration
| Body weight |
Recommended dosage form |
Loading dose (on Day 1) |
Maintenance dose (from Day 2) |
| 3 kg to less than 40 kg |
Veklury for injection, lyophilized powder Only |
5 mg/kg |
2.5 mg/kg |
Lyophilized powder: 100 mg of Veklury (remdesivir) reconstituted with 19 mL of Sterile Water for Injection. The only approved dosage form of Veklury for pediatric patients weighing 3 kg to less than 40 kg is Veklury for injection (supplied as 100 mg lyophilized powder in vial).
Further dilute to a concentration of 1.25 mg/mL using 0.9% sodium chloride.
- Small 0.9% sodium chloride infusion bags (e.g., 25, 50, or 100 mL) or an appropriately sized syringe should be used for pediatric dosing.
Recommended Rate of Infusion-Diluted Veklury for Injection Lyophilized Powder for Pediatric Patients Weighing 3 kg to Less than 40 kg
| Infusion volume |
Infusion time1 |
Rate of infusion |
| 100 mL |
30 min |
3.33 mL/min |
| 60 min |
1.67 mL/min |
| 120 min |
0.83 mL/min |
| 50 mL |
30 min |
1.67 mL/min |
| 60 min |
0.83 mL/min |
| 120 min |
0.42 mL/min |
| 25 mL |
30 min |
0.83 mL/min |
| 60 min |
0.42 mL/min |
| 120 min |
0.21 mL/min |
| 7 mL |
30 min |
0.23 mL/min |
| 60 min |
0.12 mL/min |
| 120 min |
0.06 mL/min |
Operational Considerations for Outpatient Veklury Administration
Infusion schedule and hours of operation:
- Infusion requires 3 consecutive days of administration
- Infusion sites need to have appropriate hours of operation to accommodate complete infusion cycle
Pediatric-specific infusion:
- Providers credentialed in pediatrics
- Staff trained in assessment and management of pediatric patients
Capacity for lab testing, liver function, and prothrombin time as clinically appropriate
Post-Veklury Administration Observation
- Per Veklury prescribing information, "Monitor patients during dose administration and observe for at least 1 hour after intravenous infusion or subcutaneous dosing is complete".
- Provide education on follow-up, required isolation per CDC guidelines after COVID-19 exposure or diagnosis, red flags for seeking emergency care.
- Respond to severe adverse events/anaphylaxis.
- "Discharge" patient after one-hour post-administration observation if stable and without symptoms of severe adverse reaction, otherwise consider further observation or emergency department evaluation if clinical concern.
- To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or MedWatch
For more information, see:
Managing Adverse Reactions to Veklury
Veklury should only be administered in settings in which health care providers have immediate access to medications (e.g., epinephrine) to treat a severe infusion or hypersensitivity reactions, such as anaphylaxis, and the ability to activate the emergency medical system (EMS), as necessary.
Early identification of anaphylaxis. Symptoms may include:
- Respiratory: hypoxia, dyspnea, wheezing, angioedema
- Gastrointestinal: nausea, transaminase elevation
- Cardiovascular: hypotension, hypertension, tachycardia, bradycardia
- Skin/mucosal: rash
- Neurologic: agitation, convulsions, altered mental status, sense of impending doom
- Other: diaphoresis, shivering, fever
Managing Adverse Reactions to Infused Outpatient COVID Therapeutics: Medications and Equipment
Should be available at all sites:
- Epinephrine (e.g., prefilled syringe or autoinjector)
- H1 antihistamine (e.g., diphenhydramine, cetirizine)
- Blood pressure monitor
If feasible, include at sites (not required):
- Pulse Oximeter
- Oxygen
- Bronchodilator (e.g., albuterol)
- H3 antihistamine (e.g., famotidine, cimetidine)
- Intravenous fluids
- Intubation kit
- Pocket mask with one-way valve (CPR mask) sized for adults and children
This list is adapted from CDC Interim Considerations: Preparing for the Potential Management of Anaphylaxis at COVID-19 Vaccine Sites
Veklury Storage and Preparation
Administration preparation process:
- Prepare sterile infusions in a manner consistent with local laws, regulations, guidelines and policies
- Obtain new vial(s) and/or IV bags if the drug product contains any visible particulate matter
- See Prescribing Information for reconstitution instructions
Needs for space to prepare infusion:
- Dedicated preparation area for sterile preparation
Acceptable equipment for Veklury drug storage:
- Refrigerated storage (2-8 °C)
- Temperature control mechanism including temperature monitoring process
Note: product can be prepared for infusion bedside by any qualified medical professional
For more information visit the Official Veklury Website
Additional Veklury Product Information
FDA approved for certain COVID19 indications, outpatient and inpatient
Veklury Procurement and Ordering:
Veklury is commercially available through Gilead Pharmaceuticals.
Veklury is available through multiple distributors.
- Outpatient distribution: Amerisource Bergen and Cardinal
- Non-hospital entities that can attest to the proper administration of Veklury in accordance with the label can order Veklury for outpatient use.
- Inpatient hospital distribution: Hospitals should continue ordering Veklury through AmerisourceBergen Specialty Division, Cardinal Specialty, and McKesson Plasma & Biologics.
Treatment Locator Tool include outpatients Veklury (remdesivir) providers that allows visibility of Veklury outpatient infusion sites on the Treatments Locator (hhs.gov) to assist in matching patients at high risk of severe COVID-19 to the medications that can prevent disease progression.
Infusion sites can opt in using this simple online form. All that is needed from provider is location information and agreement to participate)
- For provider sites that want to be removed from the locator, follow the opt in link, fill out the form and select to opt out before re-submitting. In order to opt out, please ensure you use the EXACT information previously used when provider opted in.
Sites are encouraged to offer outpatient Veklury, especially in collaboration with tertiary centers treating immunocompromised patients for whom Paxlovid may not be clinically appropriate (e.g., transplant centers, oncology, etc.) and for vulnerable pediatric patients not eligible for other outpatient treatments.
Example of Patient Flow for Outpatient Intravenous Product
 Pre-treatment
Pre-treatment
Confirm diagnosis of COVID-19 infection
Discuss treatment with patient
- Ensure patient meets treatment requirements and understands risks.
Schedule the patient to come in for treatment ASAP (3 consecutive days for Veklury)
- Provide guidance on site visit protocols to patients.
- Provide patient education on what to expect with administration.
Pre-treatment steps should be completed via telemedicine as possible (~30 mins)
 Treatment
Treatment
Pre-book time for administration space and follow clear protocol for coming onsite
- Ensure operationally ready to receive and treat the patient.
- Use CDC recommended practices to minimize exposure to others.
Provide treatment to patient
- Infusion duration up to ~1 hr* with an additional 1 hr of observation post infusion (checks during infusion and observation).
- Infusion pumps or gravity-based infusion acceptable.
*Contingent on product dilution, reference EUA fact sheet or prescribing information for dilution and infusion timing
Ensure preparation for administration reactions as unlikely but possible side effect
- Infusion rate may be reduced based on patient circumstances.
- Ensure emergency action plan in place; ability to activate EMS if necessary, a requirement for administration under EUA.
 Post-treatment
Post-treatment
Discharge patient immediately following monitoring completion
- Follow clear protocol to minimize risk of exposure to others
- Schedule subsequent appointments for Veklury and ensure patient understands importance of completing medication course
Post-treatment care encouraged to be via telemedicine as possible
- Normal follow-up care, no special data tracking requirements
Lagevrio (molnupiravir)
Merck Oral Antiviral (Alternative)
Lagevrio is an alternative therapy product for treatment of mild to moderate COVID-19:
Lagevrio Product Information
FDA Fact Sheets
Manufacturer's Resources
Safety Reporting
Lagevrio Authorization
Lagevrio has been authorized by FDA under an EUA, for the treatment of mild to moderate COVID-19 in adults who are at high-risk for progression to severe COVID-19, including hospitalization or death, for whom alternative COVID-19 outpatient treatment options approved or authorized by FDA are not accessible or clinically appropriate.
Lagevrio is not authorized for:
- Patients less than 18 years of age
- Initiation of treatment in patients requiring hospitalization due to COVID-19
- Use longer than 5 consecutive days
Lagevrio (molnupiravir) may only be prescribed for an individual patient by physicians, advanced practice registered nurses, and physician assistants that are licensed or authorized under state law to prescribe drugs in the therapeutic class to which Lagevrio belongs (i.e., anti-infectives).
Dosage and Administration
- 800 mg (four 200 mg capsules) taken orally every 12 hours for 5 days, with or without food.
- Take Lagevrio as soon as possible after a diagnosis of COVID-19 has been made, and within 5 days of symptom onset.
- Lagevrio is not authorized for use for longer than 5 consecutive days.
- For administration via nasogastric (NG) or orogastric (OG) Tube (12F or larger), refer to instructions within the Lagevrio provider fact sheet, Section 2.3.
Contraindications and Precautions
- No contraindications have been identified based on the limited available data on the emergency use of Lagevrio authorized under the EUA.
- Not recommended for use during pregnancy and not authorized for use in patients under 18 years of age.
- Hypersensitivity reactions, including anaphylaxis have been reported with Lagevrio.
- If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue Lagevrio (molnupiravir).
Lagevrio Checklist Tool for Prescribers
For more information see the Molnupiravir Checklist Tool for Prescribers
Lagevrio Patient Eligibility
*Prescriber is encouraged to include a note to the pharmacist in the prescription stating:
Please fill prescription by __________[insert date]_________. This prescription fill by date is within 5 days from symptom onset and complies with the patient eligibility criteria under the EUA.
Lagevrio Prescriber Requirements for All Patients
- Provide electronic or hard copy of patient fact sheet
- Document that patient has received an electronic or hard copy of the patient fact sheet.
- How and where documentation occurs is at the discretion of the prescribing health care provider and their clinical site.
- Review the information contained within the patient factsheet with the patient and counsel patient on the known and potential benefits and risks of Lagevrio.
- Advise patients on need for contraception use as appropriate.
- Females of childbearing potential treated: should use a reliable method of contraception correctly and consistently, as applicable, for the duration of treatment and for 4 days after the last dose of Lagevrio.
- Males of reproductive potential treated: if sexually active with females of childbearing potential, should use a reliable method of contraception correctly and consistently during treatment and for at least 3 months after the last dose.
- The prescribing healthcare provider and/or the provider's designee must report all medication errors and serious adverse events potentially related to Lagevrio within 7 calendar days from the healthcare provider's awareness of the event.
Lagevrio Prescriber Requirements for Individuals of Childbearing Potential
Assess whether pregnant (or not).
- Report of LMP in an individual who has regular menstrual cycles, uses a reliable method of contraception correctly and consistently or has had a negative pregnancy test
- Negative pregnancy test (recommended but not required if other criteria are not met)
If pregnant:
- Counsel the patient regarding the known and potential benefits and potential risks of molnupiravir use during pregnancy.
- Document that the patient is aware of the known and potential benefits and potential risks of molnupiravir use during pregnancy.
- Make the individual aware of the pregnancy registry at https://covidpr.pregistry.com or 1-800-616-3791
If not pregnant:
- Make the individual aware of the pregnancy registry program and encourage them to participate should they become pregnant.
Viral RNA Rebound
- Viral RNA Rebound Post-treatment increases in SARS-CoV-2 RNA shedding levels (i.e., viral RNA rebound) in nasopharyngeal samples were observed on Day 10, Day 15, and/or Day 29 in a subset of Lagevrio and placebo recipients in the Phase 3 MOVe-OUT trial. Approximately 1% of both Lagevrio and placebo recipients had evidence of recurrent COVID-19 symptoms coinciding with a rebound in viral RNA levels in nasopharyngeal samples.
- Post-treatment viral RNA rebound was not associated with the primary clinical outcome of hospitalization or death through Day 29 following the single 5-day course of Lagevrio treatment. Post-treatment viral RNA rebound also was not associated with the detection of cell culture infectious virus in nasopharyngeal swab samples.
- Viral RNA rebound* is not specific to Lagevrio or Paxlovid and has been seen in placebo recipients.
* Not exclusive to Lagevrio
For more information, see the section called Microbiology (12.4) in the Fact Sheet for Healthcare Providers: EUA for Lagevrio
Lagevrio Product Information & Ordering Logistics

